
Chemistry, 24.03.2020 19:56 tiannahwlit
G For the following reaction, 0.500 moles of silver nitrate are mixed with 0.285 moles of copper(II) chloride. What is the formula for the limiting reagent? What is the maximum amount of silver chloride that can be produced?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 2

Chemistry, 22.06.2019 05:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change
Answers: 3

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 22.06.2019 16:00
Inside a flashbulb, oxygen surrounds a thin coil of magnesium. when the flashbulb is set off, a chemical reaction takes place in which magnesium combines with oxygen to form magnesium oxide. which of the chemical equations matches the reaction above? a. mg + o2 mgo2 + energy b. 2mg + o mg2o + energy c. 2mg + o2 2mgo + energy d. mg + o mgo + energy
Answers: 1
You know the right answer?
G For the following reaction, 0.500 moles of silver nitrate are mixed with 0.285 moles of copper(II)...
Questions







Mathematics, 13.03.2021 01:40


English, 13.03.2021 01:40


Mathematics, 13.03.2021 01:40


Mathematics, 13.03.2021 01:40




Mathematics, 13.03.2021 01:40

History, 13.03.2021 01:40

History, 13.03.2021 01:40

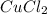 is the formula for the limiting reagent.
is the formula for the limiting reagent.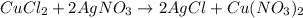
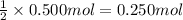 of copper(II) chloride
of copper(II) chloride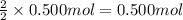 of silver chloride
of silver chloride

