Chemistry, 24.03.2020 20:55 juancarlosguevpdppo5
Given the following reaction:
NaCl + AgNO3 → AgCl + NaNO3
How many grams of AgCl will be produced from 7.00 g of NaCl and 95.0 g of AgNO3?

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 10:00
Ill give brainiestif one neutron initiates a fission event that produces two neutrons in the products, how many new reactions can now be initiated? if each of the neutrons produced in the first fission event then initiates a fission event that produces one neutron in the products, how many new reactions can now be initiated by each neutron? how many neutrons in total were produced by the two fission events described?
Answers: 2
You know the right answer?
Given the following reaction:
NaCl + AgNO3 → AgCl + NaNO3
How many grams of AgCl will be p...
NaCl + AgNO3 → AgCl + NaNO3
How many grams of AgCl will be p...
Questions

Mathematics, 25.08.2019 02:50


Mathematics, 25.08.2019 02:50

Physics, 25.08.2019 02:50




Chemistry, 25.08.2019 02:50

English, 25.08.2019 02:50


Mathematics, 25.08.2019 02:50

Mathematics, 25.08.2019 02:50

Social Studies, 25.08.2019 02:50

World Languages, 25.08.2019 02:50

Biology, 25.08.2019 02:50




Mathematics, 25.08.2019 02:50

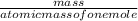 1 equation
1 equation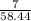
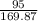
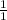 =
=