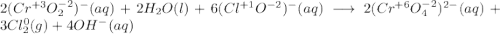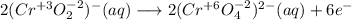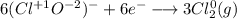Chemistry, 24.03.2020 20:58 grayjasmine46
Consider the following balanced redox reaction: 2CrO2-(aq) + 2H2O(l) + 6ClO-(aq) LaTeX: \longrightarrow⟶ 2CrO42-(aq) + 3Cl2(g) + 4OH-(aq) 1. Which species is being oxidized? 2. Which species is being reduced? 3. Which species is the oxidizing agent? 4. Which species is the reducing agent? 5. How many electrons are being transferred? Hint: If you were to balance this equation how many electrons would be in each half-reaction? That is how many electrons are transferred.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Of the groups of elements below, which are most likely to gain electrons to become anions? a. alkali metal b. boron group c. halogen d. transition metal
Answers: 2

Chemistry, 22.06.2019 12:10
|using the periodic tablewarm-upuse the periodic table in the tools bar to answer the following questions.what elemental classification does oxygen belongto? done
Answers: 3

Chemistry, 22.06.2019 15:30
Which statement names the physical property of wood a. wood is softer than coal b. wood does not rust c. wood can rot d. wood can burn
Answers: 1

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1
You know the right answer?
Consider the following balanced redox reaction: 2CrO2-(aq) + 2H2O(l) + 6ClO-(aq) LaTeX: \longrightar...
Questions

Physics, 03.02.2020 04:49


Spanish, 03.02.2020 04:49


Arts, 03.02.2020 04:49

Mathematics, 03.02.2020 04:49




Mathematics, 03.02.2020 04:49

Mathematics, 03.02.2020 04:49


Social Studies, 03.02.2020 04:49


Mathematics, 03.02.2020 04:49

Social Studies, 03.02.2020 04:49


Mathematics, 03.02.2020 04:49