Chemistry, 24.03.2020 21:15 elizzabel1944
Consider the system at equilibrium. PCl 5 ( g ) − ⇀ ↽ − PCl 3 ( g ) + Cl 2 ( g ) How will increasing the concentration of PCl5 shift the equilibrium? to the left to the right no effect How will increasing the concentration of PCl3 shift the equilibrium? to the right no effect to the left How will increasing the pressure by adding argon gas to the reaction mixture, while maintaining a constant volume, shift the equilibrium? to the right to the left no effect

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Perform the following mathematical operations and report the answer to the appropriate number of significant figures 5.87998 + 3.100
Answers: 2


Chemistry, 22.06.2019 04:30
Use the drop-down menus to answer each question. which runner finished the 100 m race in the least amount of time? which runner stopped running for a few seconds during the race? at what distance did anastasia overtake chloe in the race?
Answers: 1

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1
You know the right answer?
Consider the system at equilibrium. PCl 5 ( g ) − ⇀ ↽ − PCl 3 ( g ) + Cl 2 ( g ) How will increasing...
Questions



History, 05.10.2019 02:00

Mathematics, 05.10.2019 02:00


Mathematics, 05.10.2019 02:00

Mathematics, 05.10.2019 02:00





Physics, 05.10.2019 02:00

Mathematics, 05.10.2019 02:00


Health, 05.10.2019 02:00



Physics, 05.10.2019 02:00


Social Studies, 05.10.2019 02:00

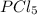 shift the equilibrium to the right side.
shift the equilibrium to the right side.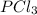 shift the equilibrium to the left.
shift the equilibrium to the left.