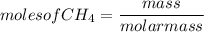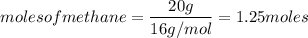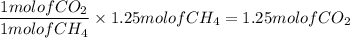Chemistry, 24.03.2020 21:11 cyaransteenberg
Given the following reaction:
CH4 +202
CO2 + 2H2O
How many moles of CO2, will be produced from 20.0 g of CH4, assuming O2 is available in excess?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Energy is released during which phase changes? check all that apply. boiling condensing depositing freezing melting subliming
Answers: 2

Chemistry, 21.06.2019 18:30
A6.10 m nacl can be made by adding [x]g of nacl to a container and making the volume of water up to the 1.00 l line
Answers: 1

Chemistry, 22.06.2019 08:30
Sally is making a model of a magnesium atom with an atomic mass number of 24 for her chemistry class. she has foam balls for the protons, neutrons, and electrons. she has added 6 neutrons to her model so far. how many more neutrons does she need to add to complete her neutral atom of magnesium?
Answers: 1

Chemistry, 23.06.2019 11:30
Which of the following is the most likeley example of an favorable mutation a. a mutation that makes a rabbit able run faster b. a mutation that changes the rabbit's fur to bright orange c. a mutation that changes the color of the rabbit's eyes d. a mutation that gives a rabbit a third ear
Answers: 1
You know the right answer?
Given the following reaction:
CH4 +202
CO2 + 2H2O
How many moles of CO2, will be prod...
CH4 +202
CO2 + 2H2O
How many moles of CO2, will be prod...
Questions



Mathematics, 24.02.2021 02:00

Mathematics, 24.02.2021 02:00

Mathematics, 24.02.2021 02:00

Social Studies, 24.02.2021 02:00

Mathematics, 24.02.2021 02:00


Geography, 24.02.2021 02:00

Mathematics, 24.02.2021 02:00

Chemistry, 24.02.2021 02:00



Mathematics, 24.02.2021 02:00

English, 24.02.2021 02:00

Biology, 24.02.2021 02:00

History, 24.02.2021 02:00


Mathematics, 24.02.2021 02:00

Biology, 24.02.2021 02:00