Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
What is the result of multiplying (2.5 × 1010) × (2.0 × 10-7)? a. 5.0 × 103 b. 5.0 × 10-3 c. 5.0 × 1017 d. 5.0 × 10-17
Answers: 1

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1
You know the right answer?
At a certain temperature, the equilibrium constant, K c , is 0.00376 for the reaction Cl 2 ( g ) − ⇀...
Questions

Mathematics, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01

Chemistry, 20.10.2020 04:01

Health, 20.10.2020 04:01

Mathematics, 20.10.2020 04:01





Mathematics, 20.10.2020 04:01


Mathematics, 20.10.2020 04:01




Chemistry, 20.10.2020 04:01


History, 20.10.2020 04:01


English, 20.10.2020 04:01

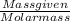
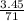
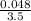 M = 0.013 molar
M = 0.013 molar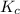 =
= ![\frac{[Cl]^{2} }{[Cl_{2} ]}](/tpl/images/0561/4874/ed9f2.png)