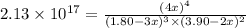Chemistry, 24.03.2020 21:33 BatmanVS1944
At a certain temperature, this reaction establishes an equilibrium with the given equilibrium constant, Kc. 3 A ( g ) + 2 B ( g ) − ⇀ ↽ − 4 C ( g ) K c = 2.13 × 10 17 If, at this temperature, 1.80 mol of A and 3.90 mol of B are placed in a 1.00 L container, what are the concentrations of A, B, and C at equilibrium?

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

Chemistry, 22.06.2019 20:20
Which formula equation represents the burning of sulfur to produce sulfur dioxide? s(s) + o2(g) 4502(9) 2h2s(s) + 302(g) —> 2h20(0) + 2502(9) 4fes2+1102 —> 2fe2o3 + 8502 2802(g) + o2(9) v205 , 2503(9)
Answers: 1
You know the right answer?
At a certain temperature, this reaction establishes an equilibrium with the given equilibrium consta...
Questions

Physics, 21.12.2019 20:31

Physics, 21.12.2019 20:31

Social Studies, 21.12.2019 20:31


Mathematics, 21.12.2019 20:31

History, 21.12.2019 20:31

Mathematics, 21.12.2019 20:31

Mathematics, 21.12.2019 20:31


Mathematics, 21.12.2019 20:31

Mathematics, 21.12.2019 20:31

Social Studies, 21.12.2019 20:31



Mathematics, 21.12.2019 20:31

Business, 21.12.2019 20:31

Health, 21.12.2019 20:31

Mathematics, 21.12.2019 20:31


![[A]=\frac{1.80 mol}{1.00 L}=1.80 M](/tpl/images/0561/5634/418e6.png)
![[B]=\frac{3.90 mol}{1.00 L}=3.90 M](/tpl/images/0561/5634/3de81.png)
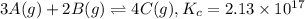
![K_c=\frac{[C]^4}{[A]^3[B]^2}](/tpl/images/0561/5634/6b95d.png)