

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2


Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1
You know the right answer?
The rate constant for the decomposition reaction of H2O2 is 3.66 × 10−3 s−1 at a particular temperat...
Questions

Biology, 30.06.2019 11:50


Mathematics, 30.06.2019 11:50



Mathematics, 30.06.2019 11:50




English, 30.06.2019 11:50

Mathematics, 30.06.2019 11:50

Physics, 30.06.2019 11:50



History, 30.06.2019 11:50




Mathematics, 30.06.2019 11:50

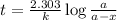
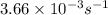
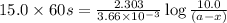
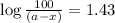
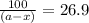
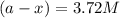
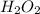 in a solution after 15.0 minutes have passed is 3.72 M
in a solution after 15.0 minutes have passed is 3.72 M


