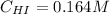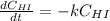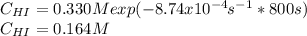Consider this reaction:
2HI(g) → H2(g)+ I2(g)
At a certain temperature it obeys t...

Consider this reaction:
2HI(g) → H2(g)+ I2(g)
At a certain temperature it obeys this rate law.
Rate= 8.74 x 10^-4 s^1
Suppose a vessel contains HI at a concentration of 0.330M. Calculate the concentration of HI in the vessel 800 seconds later. You may assume no other reaction is important. Round your answer to significant digit

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:00
Which theory was contradicted by experiments with the photoelectric effect? light spreads out after it passes through a small opening. as soon as light strikes metal, electrons will be ejected. visible light, regardless of color, will cause the ejection of electrons when striking metal. the kinetic energy of ejected electrons depends on the frequency of light that strikes the metal.
Answers: 2

Chemistry, 22.06.2019 15:30
Light waves can move through , but they travel fastest when they move through a(n) .
Answers: 1

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1
You know the right answer?
Questions



Biology, 07.07.2019 21:20

Biology, 07.07.2019 21:20






History, 07.07.2019 21:20

Mathematics, 07.07.2019 21:20

Mathematics, 07.07.2019 21:20


Mathematics, 07.07.2019 21:20

Mathematics, 07.07.2019 21:20


Chemistry, 07.07.2019 21:20

Advanced Placement (AP), 07.07.2019 21:20

Mathematics, 07.07.2019 21:20