
The dimerization of butadiene 2C4H61 g2h C8H121 g2 was studied at 500. K, and the following data were obtained: Time (s) [C4H6] (mol/L) 195 1.6 3 1022 604 1.5 3 1022 1246 1.3 3 1022 2180 1.1 3 1022 6210 0.68 3 1022 Assuming that Rate 52 D3C4H64 Dt determine the form of the rate law, the integrated rate law, and the value of the rate constant for this reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 09:00
What is the percentage composition of carbon in the compound ch4
Answers: 1


Chemistry, 22.06.2019 19:30
Astring vibrates with a frequency of 10 hz. why can't a person hear the sound waves produced by the vibrating string, no matter how large the amplitude of the waves? out! this is homework and due tomorrow! you so much!
Answers: 2
You know the right answer?
The dimerization of butadiene 2C4H61 g2h C8H121 g2 was studied at 500. K, and the following data wer...
Questions

Mathematics, 27.09.2021 06:40

Mathematics, 27.09.2021 06:40






Business, 27.09.2021 06:40





Social Studies, 27.09.2021 06:40

Physics, 27.09.2021 06:40

Mathematics, 27.09.2021 06:40


Mathematics, 27.09.2021 06:40

Mathematics, 27.09.2021 06:40


![k[C_4H_6]^2](/tpl/images/0561/6934/d2fdb.png)
![\frac{1}{[C_4H_6]}=\frac{1}{[C_4H_6]_0}+kt](/tpl/images/0561/6934/38768.png)
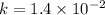
![\frac{1}{[C_4H_6]}](/tpl/images/0561/6934/fd07e.png) and the reaction is second order hence we get the rate law from
and the reaction is second order hence we get the rate law from ![k[A]^n](/tpl/images/0561/6934/3c428.png) .
. ![\frac{1}{[A]}=\frac{1}{[A]_0} +kt](/tpl/images/0561/6934/aad88.png) where A is
where A is 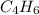 .
. 

