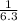Chemistry, 24.03.2020 23:12 ShelleyPSeliger
For the chemical equation SO 2 ( g ) + NO 2 ( g ) − ⇀ ↽ − SO 3 ( g ) + NO ( g ) the equilibrium constant at a certain temperature is 3.10 . At this temperature, calculate the number of moles of NO 2 ( g ) that must be added to 2.30 mol SO 2 ( g ) in order to form 1.00 mol SO 3 ( g ) at equilibrium.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 13:00
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2
You know the right answer?
For the chemical equation SO 2 ( g ) + NO 2 ( g ) − ⇀ ↽ − SO 3 ( g ) + NO ( g ) the equilibrium cons...
Questions


Advanced Placement (AP), 09.04.2020 01:39




History, 09.04.2020 01:39






English, 09.04.2020 01:40







Mathematics, 09.04.2020 01:40

Mathematics, 09.04.2020 01:40

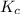 =
= ![\frac{[SO_{3} ][NO]}{[SO_{2}][NO_{2} ]}](/tpl/images/0561/8606/bc0fb.png)
![\frac{(1.00)(1.00)}{(2.30) [NO_{2} ]}](/tpl/images/0561/8606/3f85b.png) At equilibrium [SO₃] = [NO]
At equilibrium [SO₃] = [NO]