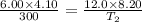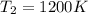Chemistry, 24.03.2020 23:00 charlesiarenee0
One mole of oxygen gas is at a pressure of 6.00 atm and a temperature of 27.0°C. (a) If the gas is heated at constant volume until the pressure triples, what is the fi nal temperature? (b) If the gas is heated so that both the pressure and volume are doubled, what is the fi nal temperature?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2


Chemistry, 22.06.2019 16:50
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
You know the right answer?
One mole of oxygen gas is at a pressure of 6.00 atm and a temperature of 27.0°C. (a) If the gas is h...
Questions


Mathematics, 01.07.2019 11:30


Computers and Technology, 01.07.2019 11:30


Chemistry, 01.07.2019 11:30



History, 01.07.2019 11:30

Biology, 01.07.2019 11:30

Mathematics, 01.07.2019 11:30


History, 01.07.2019 11:30


Mathematics, 01.07.2019 11:30

English, 01.07.2019 11:30

Mathematics, 01.07.2019 11:30


Mathematics, 01.07.2019 11:30

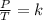
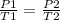
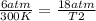
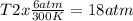
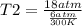
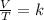
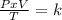
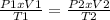
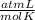 × 300 K
× 300 K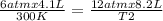
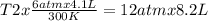
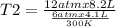
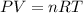
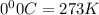
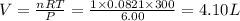
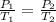
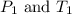 are the initial pressure and temperature of the gas.
are the initial pressure and temperature of the gas.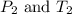 are the final pressure and temperature of the gas.
are the final pressure and temperature of the gas.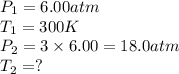
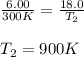
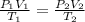
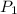 = initial pressure of gas = 6.00 atm
= initial pressure of gas = 6.00 atm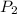 = final pressure of gas =
= final pressure of gas = 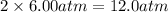
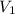 = initial volume of gas = 4.10 L
= initial volume of gas = 4.10 L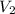 = final volume of gas =
= final volume of gas = 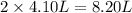
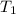 = initial temperature of gas =
= initial temperature of gas = 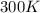
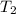 = final temperature of gas =?
= final temperature of gas =?