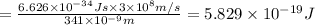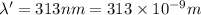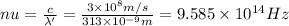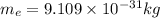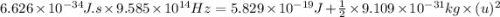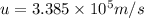The mathematical equation for studying the photoelectric effect is:
hν = W + 1/2 meμ^2...

The mathematical equation for studying the photoelectric effect is:
hν = W + 1/2 meμ^2 where ν is the frequency of light shining on the metal; W is the energy needed to remove an electron from the metal; and me and u are the mass and speed of the ejected electron, respectively. In an experiment, a student found that a maximum wavelength of 341 nm is needed to just dislodge electrons from a metal surface. Calculate the velocity (in m/s) of an ejected electron when the student employed light with a wavelength of 313 nm.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:30
If a 12-v battery is connected to a circuit that has a current of 3.0 a, what is the total resistance in the circuit? 36 ohms 4 ohms 0.25 ohms
Answers: 1


Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1
You know the right answer?
Questions

Health, 25.08.2021 22:10


English, 25.08.2021 22:10

Mathematics, 25.08.2021 22:10




History, 25.08.2021 22:10

Mathematics, 25.08.2021 22:10

Geography, 25.08.2021 22:10

Mathematics, 25.08.2021 22:10

English, 25.08.2021 22:10

History, 25.08.2021 22:10

Biology, 25.08.2021 22:10

English, 25.08.2021 22:10

Health, 25.08.2021 22:10


Computers and Technology, 25.08.2021 22:10

Mathematics, 25.08.2021 22:10

Mathematics, 25.08.2021 22:10

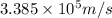 .
.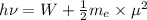
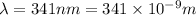
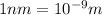
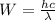 (Planck's equation)
(Planck's equation)