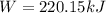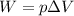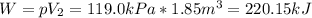Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 23.06.2019 03:00
Select the correct answer. wax is a nonpolar substance. in which type of substance is it the most soluble?
Answers: 1

Chemistry, 23.06.2019 05:40
Which order shows the levels of organization from largest to smallest? organism, organ system, cell, organ, tissue organism, tissue, organ system, organ, cell organism, organ, organ system, cell, tissue organism, organ system, organ, tissue, cell
Answers: 2
You know the right answer?
A gas‑forming reaction produces 1.85 m 3 1.85 m3 of gas against a constant pressure of 119.0 kPa. 11...
Questions


Mathematics, 18.07.2019 16:30

History, 18.07.2019 16:30

History, 18.07.2019 16:30

Mathematics, 18.07.2019 16:30



Social Studies, 18.07.2019 16:30


Mathematics, 18.07.2019 16:30



Mathematics, 18.07.2019 16:30


Mathematics, 18.07.2019 16:30

Mathematics, 18.07.2019 16:30

Advanced Placement (AP), 18.07.2019 16:30