
Chemistry, 25.03.2020 01:54 Thejollyhellhound20
What is the total volume of gaseous products formed when 116 liters of butane (C4H10) react completely according to the following reaction? (All gases are at the same temperature and pressure.) butane (C4H10) (g) + oxygen(g)carbon dioxide (g) + water(g)

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1

Chemistry, 22.06.2019 22:30
Amedication is given at a dosage of 3.000 mg of medication per kg of body weight. if 0.1500 g of medication is given, then what was the patient's weight in pounds (lbs)? there are 453.59g in 1 lb.
Answers: 2

Chemistry, 23.06.2019 04:00
Which of these are physical changes in matter? check all that apply boiling water a pencil being sharpened exploding dynamite freezing water rotting cheese
Answers: 1
You know the right answer?
What is the total volume of gaseous products formed when 116 liters of butane (C4H10) react complete...
Questions

Mathematics, 20.11.2020 21:50

Arts, 20.11.2020 21:50




Biology, 20.11.2020 21:50



Computers and Technology, 20.11.2020 21:50

English, 20.11.2020 21:50

English, 20.11.2020 21:50


Mathematics, 20.11.2020 21:50


Social Studies, 20.11.2020 21:50




Mathematics, 20.11.2020 21:50

Mathematics, 20.11.2020 21:50

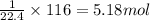 of butane
of butane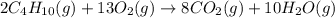
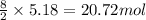 of carbon dioxide
of carbon dioxide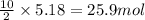 of water vapor
of water vapor

