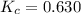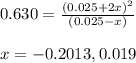Chemistry, 25.03.2020 02:08 gunnatvinson
The value of kc for the following reaction is 0.630 at 409 K N2O4(g) --> 2NO2(g) if a reaction vessel at that temperature intitially contains 0.0250 M NO2 and 0.0250 M N2O4, what is the concentration of NO2 at equilibrium

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1

Chemistry, 22.06.2019 10:40
Ammonia and oxygen react to form nitrogen monoxide and water, like this: 4nh3 (g) + 5o2 (g) → 4no (g) + 6h2o (g) also, a chemist finds that at a certain temperature the equilibrium mixture of ammonia, oxygen, nitrogen monoxide, and water has the following composition: compound pressure at equilibrium nh3 65.1atm o2 31.3atm no 62.7atm h2o 65.8atm compound pressure at equilibrium nh3 65.3 atm o2 7.79 atm no 12.1 atm h2o 65.8 atm calculate the value of the equilibrium constant kp for this reaction. round your answer to 2 significant
Answers: 2

Chemistry, 22.06.2019 12:30
Consider the four elements above. which one of these elements will combine with oxygen in a 1: 1 ratio?
Answers: 3

You know the right answer?
The value of kc for the following reaction is 0.630 at 409 K N2O4(g) --> 2NO2(g) if a reaction ve...
Questions

Mathematics, 26.06.2019 07:30


Mathematics, 26.06.2019 07:30

Mathematics, 26.06.2019 07:30

Mathematics, 26.06.2019 07:30

Mathematics, 26.06.2019 07:30

Mathematics, 26.06.2019 07:30

Mathematics, 26.06.2019 07:30




Mathematics, 26.06.2019 07:30



Mathematics, 26.06.2019 07:30

Mathematics, 26.06.2019 07:30




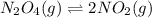
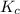 for above equation follows:
for above equation follows:![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0562/2368/271f5.png)