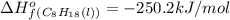Chemistry, 25.03.2020 03:05 JakkuZakku9436
Octane (C8H18) undergoes combustion according to the following thermochemical equation. 2C8H18(l) + 25O2(g) → 16CO2(g) + 18H2O(l) ΔH°rxn = –1.0940 × 104 kJ/mol What is the standard enthalpy of formation of liquid octane? ΔH°f(CO2(g)) = –393.5 kJ/mol and ΔH°f(H2O(l)) = –285.8 kJ/mol

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Complete the sentence. the lower the hydrogen ion concentration, the the ph. higher lower closer to 7 closer to 0
Answers: 2

Chemistry, 22.06.2019 12:30
Place the elements below in order of decreasing ionization energy. aluminum(al) chlorine(cl) magnesium (mg) sulfur(s)
Answers: 1

Chemistry, 22.06.2019 22:00
4.25g sample of solid ammonium nitrate dissolves in 60.0g of water in a coffee-cup calorimeter, the temperature drops from 22.0 c to 16.9 c. assume that the specific heat of the solution is the same as that of pure water. calculate delta(h) (in kj/mol nh4no3) for the solution proces.
Answers: 2

Chemistry, 23.06.2019 11:00
Find the enthalpy of neutralization of hcl and naoh. 87 cm3 of 1.6 mol dm-3 hydrochloric acid was neutralized by 87 cm3 of 1.6 mol dm-3 naoh. the temperature rose from 298 k to 317.4 k. the specific heat capacity is the same as water, 4.18 j/k g. a. -101.37 kj b. 7055 kj c. 10,1365 kj
Answers: 1
You know the right answer?
Octane (C8H18) undergoes combustion according to the following thermochemical equation. 2C8H18(l) +...
Questions




History, 20.03.2021 09:10

Arts, 20.03.2021 09:10

Mathematics, 20.03.2021 09:10

Health, 20.03.2021 09:10

Mathematics, 20.03.2021 09:10

Mathematics, 20.03.2021 09:10

Arts, 20.03.2021 09:10



Social Studies, 20.03.2021 09:20

Computers and Technology, 20.03.2021 09:20



Mathematics, 20.03.2021 09:20



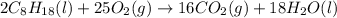
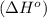 .
.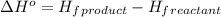
![\Delta H^o=[n_{O_2}\times \Delta H_f^0_{(O_2)}+n_{H_2O}\times \Delta H_f^0_{(H_2O)}]-[n_{C_8H_{18}}\times \Delta H_f^0_{(C_8H_{18})+n_{O_2}\times \Delta H_f^0_{(O_2)}]](/tpl/images/0562/3120/bdc44.png)
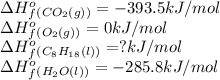
![-1.0940\times 10^4=[(16\times -393.5)+(18\times -285.8)]-[(25\times 0)+(2\times \Delat H_f{C_8H_{18}(l)}]](/tpl/images/0562/3120/9e959.png)