
Predict whether the equilibria
I) CO2(g) + 2 NH3(g) =
CO(NH2)2(s) + H2O(g),
AH° = -90 kJ
II) Ni(s) + 4 CO(g) = Ni(CO)4(8),
AH° = -161 kJ
will shift toward products or reactants with a
temperature increase.
1. I shifts toward reactants and II shifts
toward products.
2. Both I and II shift toward products.
3. Unable to determine
4. Both I and II shift toward reactants.
5. I shifts toward products and II shifts to-
ward reactants.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
You are performing an experiment in a lab to attempt a new method of producing pure elements from compounds. the only problem is that you do not know what element will form. by your previous calculations you know that you will have 6.3 moles of product. when it is complete, you weigh it and determine you have 604.4 grams. what element have you produced?
Answers: 1

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1
You know the right answer?
Predict whether the equilibria
I) CO2(g) + 2 NH3(g) =
CO(NH2)2(s) + H2O(g),
AH° =...
I) CO2(g) + 2 NH3(g) =
CO(NH2)2(s) + H2O(g),
AH° =...
Questions


Geography, 02.09.2020 05:01

Business, 02.09.2020 05:01

Mathematics, 02.09.2020 05:01

Computers and Technology, 02.09.2020 05:01










English, 02.09.2020 05:01





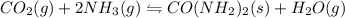 ,ΔH° = -90 kJ
,ΔH° = -90 kJ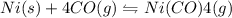 ,ΔH° = -161 kJ
,ΔH° = -161 kJ

