

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the element whose electric configuration ends in the d sublevel, the element is calssified as? a.inner transition b.noble gases c.representative d. transition
Answers: 2

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2

You know the right answer?
Consider the following reaction: 2SO2(g)+O2(g)⇌2SO3(g) Kp=0.355 at 950 K A 2.75−L reaction vessel at...
Questions







Computers and Technology, 25.12.2019 00:31








Computers and Technology, 25.12.2019 00:31





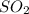 and
and 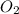
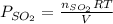
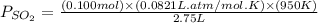
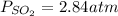
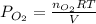
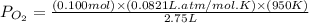
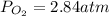
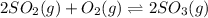
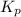 for above reaction follows:
for above reaction follows: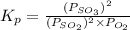
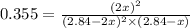
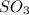 = (2x) = 2(0.664) = 1.33 atm
= (2x) = 2(0.664) = 1.33 atm

