
Chemistry, 25.03.2020 05:05 chonawilson4
Ozone (O3) in the atmosphere can be converted to oxygen gas by reaction with nitric oxide (NO). (Nitrogen dioxide is also produced in the reaction.) What is the enthalpy change when 8.50 L of ozone at a pressure of 1.00 atm and 25°C reacts with 12.00 L of nitric oxide at the same initial pressure and temperature (R = 0.0821 L atm/mol K)? [ΔH°f(NO) = 90.4 kJ/mol; ΔH°f(NO2) = 33.85 kJ/mol; ΔH°f(O3) = 142.2 kJ/mol]

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Determine the empirical formula of a compound containing 40.6 grams of carbon, 5.1 grams of hydrogen, and 54.2 grams of oxygen. in an experiment, the molar mass of the compound was determined to be 118.084 g/mol. what is the molecular formula of the compound? for both questions, show your work or explain how you determined the formulas by giving specific values used in calculations.
Answers: 3

Chemistry, 21.06.2019 23:00
The drawing represents the movement of particles in a substance. what changes of state can this substance undergo
Answers: 1

Chemistry, 21.06.2019 23:30
What’s the scientific notation for the number 6,840,000,000
Answers: 1

Chemistry, 22.06.2019 10:00
3. how much energy in joules is required to evaporate .0005 kg of liquid ammonia to vapor at the same temperature? 4. how much energy ( in megajoules ) is given up by .75 kg of water at 0c when it freezes to form ice at 0c? 5. explain how heat works between and at critical temperatures?
Answers: 2
You know the right answer?
Ozone (O3) in the atmosphere can be converted to oxygen gas by reaction with nitric oxide (NO). (Nit...
Questions





Mathematics, 09.04.2020 20:57

English, 09.04.2020 20:57


Mathematics, 09.04.2020 20:57

Chemistry, 09.04.2020 20:57






Mathematics, 09.04.2020 20:57



Social Studies, 09.04.2020 20:57


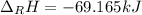
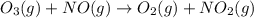
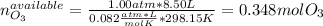
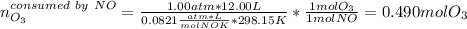
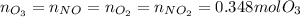
![\Delta _RH=n[\Delta _fH_{products}-\Delta _fH_{reagents}]\\\Delta _RH=0.348mol*[33.85 kJ/mol+0kJ/mol-142.2 kJ/mol-90.4 kJ/mol]\\\Delta _RH=-69.165kJ](/tpl/images/0562/4480/3a1de.png)


