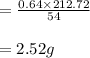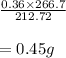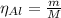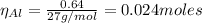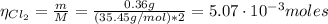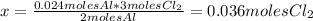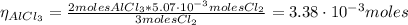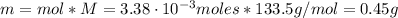Chemistry, 25.03.2020 05:47 ruchierosanp1n3qw
The balanced equation for the reaction of aluminum metal and chlorine gas is 2Al(s) + 3Cl2(g) → 2AlCl3(s) Assume that 0.64 g Al is mixed with 0.36 g Cl2. (a) What is the limiting reactant? Al Cl2 (b) What is the maximum amount of AlCl3, in grams, that can be produced?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1

Chemistry, 23.06.2019 04:40
6) (a) calculate the absorbance of the solution if its concentration is 0.0278 m and its molar extinction coefficient is 35.9 l/(mol cm). the depth of the cell is 5 mm. (b) what is the %t? (7) calculate the absorbance of the solution if the transmitted light intensity is 70% of the initial light beam intensity
Answers: 1
You know the right answer?
The balanced equation for the reaction of aluminum metal and chlorine gas is 2Al(s) + 3Cl2(g) → 2AlC...
Questions



Mathematics, 25.03.2020 21:29



Mathematics, 25.03.2020 21:29


History, 25.03.2020 21:29


English, 25.03.2020 21:29

Physics, 25.03.2020 21:29

Mathematics, 25.03.2020 21:29

Mathematics, 25.03.2020 21:29

Biology, 25.03.2020 21:30

English, 25.03.2020 21:30



English, 25.03.2020 21:30