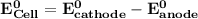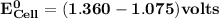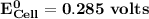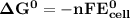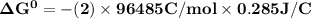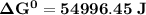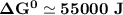Chemistry, 25.03.2020 05:26 kenzie3497
Determine ΔG° for a cell that utilizes the following reaction: Cl2(g) + 2Br–(aq) → 2Cl–(aq) + Br2(l) The standard reduction for the chlorine gas is 1.360 volts and the standard reduction for the bromine liquid is about 1.075 volts. Group of answer choices

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 07:50
Which of the following electromagnetic waves can create ions?
Answers: 2

You know the right answer?
Determine ΔG° for a cell that utilizes the following reaction: Cl2(g) + 2Br–(aq) → 2Cl–(aq) + Br2(l)...
Questions










Biology, 14.10.2020 01:01

Mathematics, 14.10.2020 01:01


Computers and Technology, 14.10.2020 01:01

Mathematics, 14.10.2020 01:01

Mathematics, 14.10.2020 01:01

Mathematics, 14.10.2020 01:01

English, 14.10.2020 01:01


Mathematics, 14.10.2020 01:01

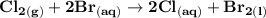
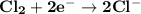
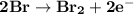
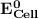 can be calculated as:
can be calculated as: