Chemistry, 25.03.2020 05:38 codyfore141
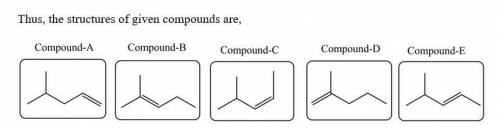
Five isomeric alkenes A–E each undergo catalytic hydrogenation to give 2-methylpentane. The IR spectra of these five alkenes have the following key absorptions (in cm–1): Compound A: 912 (s), 994 (s), 1643 (s), 3077 (m) Compound B: 833 (s), 1667 (w), 3050 (weak shoulder on C–H absorption) Compound C: 714 (s), 1665 (w), 3010 (m) Compound D: 885 (s), 1650 (m), 3086 (m) Compound E: 967 (s), no absorption 1600–1700, 3040 (m) The alkene structures are given below. Identify each compound.

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 14:30
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4.0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 22.06.2019 19:30
Use the periodic table to find the molar mass of each element. molar mass h = g/mol molar mass s = g/mol molar mass o = g/mol
Answers: 3
You know the right answer?
Five isomeric alkenes A–E each undergo catalytic hydrogenation to give 2-methylpentane. The IR spect...
Questions


Mathematics, 18.12.2020 19:50

Mathematics, 18.12.2020 19:50


Mathematics, 18.12.2020 19:50


History, 18.12.2020 19:50

Arts, 18.12.2020 19:50




English, 18.12.2020 19:50



Mathematics, 18.12.2020 19:50

English, 18.12.2020 19:50

Computers and Technology, 18.12.2020 19:50


Chemistry, 18.12.2020 19:50