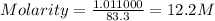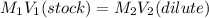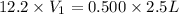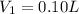Chemistry, 25.03.2020 05:31 wallsdeandre25521
Hydrochloric acid is usually purchased in concentrated form with a 37.0% HCl concentration by mass and a density of 1.20 g / mL. How much of the concentrated stock solution in milliliters should you use to make 2.5 L of 0.500 M HCl

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 23.06.2019 05:30
Based on the formulas, select the compounds below that are covalent: kbr sif4 al2o3 co2 naco3 s7o2 pcl3 fe3n2 h2o s2f10
Answers: 3

Chemistry, 23.06.2019 05:30
The term gas is limited to those substances that exist in the gaseous state at
Answers: 1
You know the right answer?
Hydrochloric acid is usually purchased in concentrated form with a 37.0% HCl concentration by mass a...
Questions

Computers and Technology, 08.07.2019 06:30

Social Studies, 08.07.2019 06:30



History, 08.07.2019 06:30

Biology, 08.07.2019 06:30



Mathematics, 08.07.2019 06:30


Biology, 08.07.2019 06:30

History, 08.07.2019 06:30



Social Studies, 08.07.2019 06:30

Chemistry, 08.07.2019 06:30



Mathematics, 08.07.2019 06:30

Geography, 08.07.2019 06:30

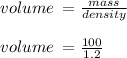
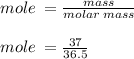
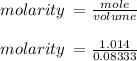
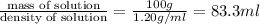
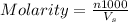
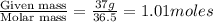
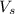 = volume of solution in ml = 83.3 ml
= volume of solution in ml = 83.3 ml