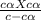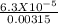Chemistry, 25.03.2020 06:39 aangellexith2885
Benzoic acid is a weak monoprotic acid. It has a pKa of 4.20. A student transferred 25.00mL of 0.126M benzoic acid into a beaker. The student then added 20.00mL of distilled water into the beaker. Calculate the equilibrium pH for this benzoic acid solution.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Which of the dna typing techniques do you think you would choose if you had to analyze a dna sample? why?
Answers: 1

Chemistry, 22.06.2019 08:30
The mass of a neutron is equal to the mass of a proton plus the mass of an electron. true or false false true
Answers: 1

Chemistry, 22.06.2019 12:00
1. if you have a gas at 127 degrees c, what is it's absolute temperature (kelvin)? a. 200kb. 300kc. 400kd. 500k2. if you had a gas whose absolute temperature measured 45 k, what is that temperature in celsius? a. -228 cb. -300 cc. 125 cd. 112 c
Answers: 2

You know the right answer?
Benzoic acid is a weak monoprotic acid. It has a pKa of 4.20. A student transferred 25.00mL of 0.126...
Questions

English, 25.01.2021 17:50


Mathematics, 25.01.2021 17:50

Health, 25.01.2021 17:50


Spanish, 25.01.2021 17:50





Mathematics, 25.01.2021 17:50



Mathematics, 25.01.2021 17:50





History, 25.01.2021 17:50

Arts, 25.01.2021 17:50