
Chemistry, 25.03.2020 16:17 jimarieb08
In Part A, you found the number of moles of product (1.80 mol P2O5 ) formed from the given amount of phosphorus and excess oxygen. In Part B, you found the number of moles of product (1.40 mol P2O5 ) formed from the given amount of oxygen and excess phosphorus. Now, determine the number of moles of P2O5 is produced from the given amounts of phosphorus and oxygen

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 23.06.2019 02:00
What is the source of continuous heat and energy that we receive from the sun
Answers: 2

Chemistry, 23.06.2019 08:30
If you had to research a particular disease or area of concern in veterinary medicine and science, which one would you choose? why?
Answers: 1

Chemistry, 23.06.2019 10:00
The temperature of a lead fishing weight rises from 26 °c to 38 °c as it absorbs 11.3 j of heat. what is the mass of the fishing weight in grams?
Answers: 1
You know the right answer?
In Part A, you found the number of moles of product (1.80 mol P2O5 ) formed from the given amount of...
Questions

Mathematics, 30.01.2020 00:50


Physics, 30.01.2020 00:50


Mathematics, 30.01.2020 00:50

Mathematics, 30.01.2020 00:50


Social Studies, 30.01.2020 00:50

History, 30.01.2020 00:50

History, 30.01.2020 00:50



French, 30.01.2020 00:50

Mathematics, 30.01.2020 00:50


Physics, 30.01.2020 00:50

Mathematics, 30.01.2020 00:50


English, 30.01.2020 00:50

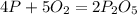
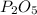 is formed by 4 moles of
is formed by 4 moles of 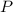
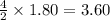
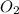 required =
required = 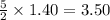
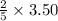 = 1.40 moles.
= 1.40 moles.


