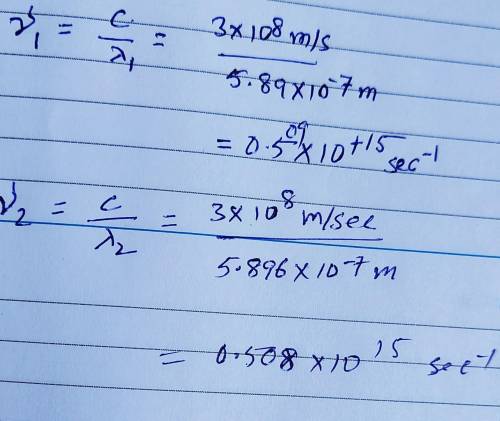Sodium vapor lamps are used to illuminate streets and highways. The very bright light emitted by these lamps is actually due to two closely spaced emission lines in the visible region of the electromagnetic spectrum. One of these lines has a wavelength of 5.890 X 10⁻⁷ m, and the other line has a wavelength of 5.896 X 10⁻⁷ m. A) What are the wavelengths of these radiations in centimeters? B) Calculate the frequencies of these radiations. Show work please

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:40
In the following table, all the columns for the element calcium are filled out correctly. element electron structure of atom electron structure of ion net ionic charge calcium 1s22s22p63s23p64s2 1s32s22p63s23p64s1 +1 true false
Answers: 2

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

Chemistry, 23.06.2019 00:00
How is the way a mixture is combined different from how a compound is combined?
Answers: 3

Chemistry, 23.06.2019 08:30
Sand is more likely than shale to preserve fossils. true false
Answers: 2
You know the right answer?
Sodium vapor lamps are used to illuminate streets and highways. The very bright light emitted by the...
Questions

Chemistry, 06.07.2021 02:00


Mathematics, 06.07.2021 02:10

Biology, 06.07.2021 02:10

Mathematics, 06.07.2021 02:10



Advanced Placement (AP), 06.07.2021 02:10


Mathematics, 06.07.2021 02:10





English, 06.07.2021 02:10



Mathematics, 06.07.2021 02:10

Mathematics, 06.07.2021 02:10

Mathematics, 06.07.2021 02:10