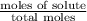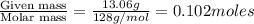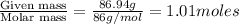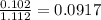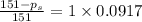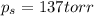Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Can you reduce your impairment by drinking coffee or cold showers? true or false?
Answers: 3

Chemistry, 22.06.2019 05:40
Why did southern business leaders want to increase the number of slaves
Answers: 1


Chemistry, 22.06.2019 19:50
Which sentence from holes contains an implied personality trait? stanley and his parents had tried to pretend that he was just going away to camp for a while, just like rich kids do. he'd just been in the wrong place at the wrong time. stanley felt somewhat dazed as the guard unlocked his handcuffs and led him off the bus. stanley nodded to show he understood
Answers: 3
You know the right answer?
A solution contains naphthalene (C10H8) dissolved in hexane (C6H14) at a concentration of 13.06% nap...
Questions

Mathematics, 17.12.2021 01:30


Mathematics, 17.12.2021 01:30

Mathematics, 17.12.2021 01:30

Mathematics, 17.12.2021 01:30






Mathematics, 17.12.2021 01:30

Mathematics, 17.12.2021 01:30

Business, 17.12.2021 01:30

English, 17.12.2021 01:30


Mathematics, 17.12.2021 01:30

Geography, 17.12.2021 01:30

Mathematics, 17.12.2021 01:30


Biology, 17.12.2021 01:30

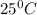 is 137 torr
is 137 torr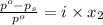
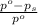 = relative lowering in vapor pressure
= relative lowering in vapor pressure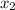 = mole fraction of solute =
= mole fraction of solute =