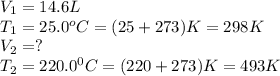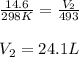Chemistry, 25.03.2020 18:00 littletiger4867
A sample of gas in a 14.6 L flexible container is at 25.0oC and 1.00atm. What is the volume of the sample when heated to 220.0oC and the pressure is constant? Remember that ALL TEMPERATURES MUST BE IN KELVIN! First: we add 273 convert Celsius to Kelvin so 25.0oC becomes 298 K We have constant pressure, two temperatures and one volume so we will solve for V2

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:40
Which type of precipitation would most likely form when the surface air temperature is slightly below freezing and the air temperature increases as you move upward away from the ground?
Answers: 2

Chemistry, 22.06.2019 12:00
Which of the following units is not an official si unit? mole liter kilogram ampere
Answers: 1

Chemistry, 22.06.2019 19:30
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 22.06.2019 20:10
Insoluble sulfide compounds are generally black in color. which of the following combinations could yield a black precipitate? check all that apply. na2s(aq)+kcl(aq) li2s(aq)+pb(no3)2(aq) pb(clo3)2(aq)+nano3(aq) agno3(aq)+kcl(aq) k2s(aq)+sn(no3)4(aq)
Answers: 1
You know the right answer?
A sample of gas in a 14.6 L flexible container is at 25.0oC and 1.00atm. What is the volume of the s...
Questions

Business, 30.03.2021 14:00

Mathematics, 30.03.2021 14:00

Biology, 30.03.2021 14:00

World Languages, 30.03.2021 14:00




Spanish, 30.03.2021 14:00

Mathematics, 30.03.2021 14:00

English, 30.03.2021 14:00

Mathematics, 30.03.2021 14:00

Mathematics, 30.03.2021 14:00

Computers and Technology, 30.03.2021 14:00






World Languages, 30.03.2021 14:00

Mathematics, 30.03.2021 14:00

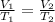
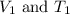 are the initial volume and temperature of the gas.
are the initial volume and temperature of the gas.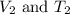 are the final volume and temperature of the gas.
are the final volume and temperature of the gas.