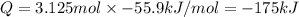125 grams of solid sodium hydroxide is combined with an excess of aqueous hydrochloric acid. The heat of neutralization for the formation of water -55.9 kJ/mole. The heat of solvation (it might be called the heat of solution) for sodium hydroxide is 41.0 kJ/mole What quantity of heat is released?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
List the two type of transporst that the cell in orde to transport molecules acroos the membrane
Answers: 1

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 04:10
Answer from each drop-down menu. e characteristics of a borane molecule (bh). the lewis structure and table of electronegativities are given olecular shape is and the molecule is reset next erved. search e a
Answers: 2

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1
You know the right answer?
125 grams of solid sodium hydroxide is combined with an excess of aqueous hydrochloric acid. The hea...
Questions

Physics, 09.04.2021 01:00


Mathematics, 09.04.2021 01:00

Mathematics, 09.04.2021 01:00

History, 09.04.2021 01:00


History, 09.04.2021 01:00


Health, 09.04.2021 01:00

Mathematics, 09.04.2021 01:00


Computers and Technology, 09.04.2021 01:00

Mathematics, 09.04.2021 01:00

Mathematics, 09.04.2021 01:00

Computers and Technology, 09.04.2021 01:00

Mathematics, 09.04.2021 01:00


Computers and Technology, 09.04.2021 01:00


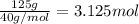

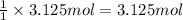 of water
of water