Given the balanced equation representing a reaction:
2HCl(aq) + Na2S2O3(aq)--> S(s) +...

Chemistry, 25.03.2020 20:19 ashley232323
Given the balanced equation representing a reaction:
2HCl(aq) + Na2S2O3(aq)--> S(s) + H2SO3(aq) + 2NaCl(aq)
Decreasing the concentration of Na2S2O3(aq) decreases the rate of reaction
because the
(1) activation energy decreases
(2) activation energy increases
(3) frequency of effective collisions decreases
(4) frequency of effective collisions increases

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
The big bang nucleosynthesis theory states that elements were produced in the first few minutes of the big bang while elements have their origins in the interiors of stars, forming much later in the history of the universe.
Answers: 1

Chemistry, 22.06.2019 12:30
If 22.5 liters of oxygen reacted with excess of hydrogen, how many liters of water vapor could be produced?
Answers: 3

Chemistry, 22.06.2019 17:00
What is the approximate vapor pressure when the gas condenses at 70 degrees celsius
Answers: 2

Chemistry, 23.06.2019 01:10
A5.00 g of a in . g of at aa 5.00 g of b in . g of .?at .
Answers: 1
You know the right answer?
Questions


Mathematics, 14.04.2021 23:00

Social Studies, 14.04.2021 23:00

Health, 14.04.2021 23:00

Arts, 14.04.2021 23:00

English, 14.04.2021 23:00


Business, 14.04.2021 23:00


Chemistry, 14.04.2021 23:00

Mathematics, 14.04.2021 23:00



Mathematics, 14.04.2021 23:00


History, 14.04.2021 23:00




Social Studies, 14.04.2021 23:00

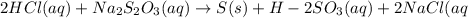
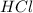 and
and 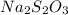 in the given case.
in the given case.

