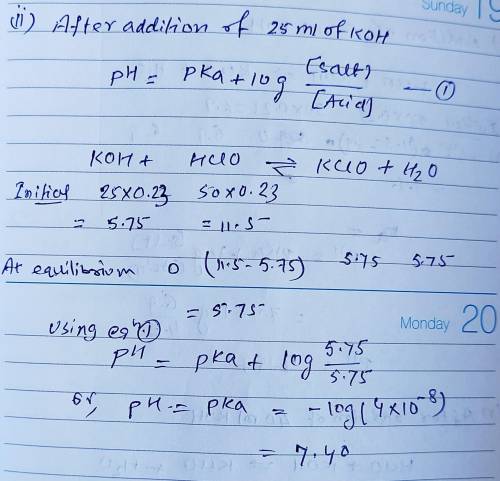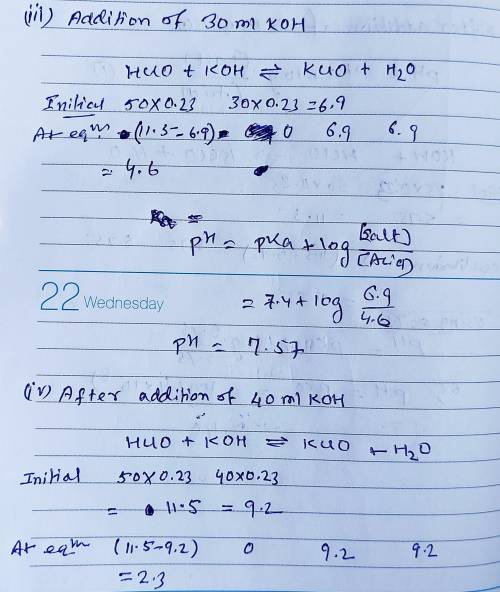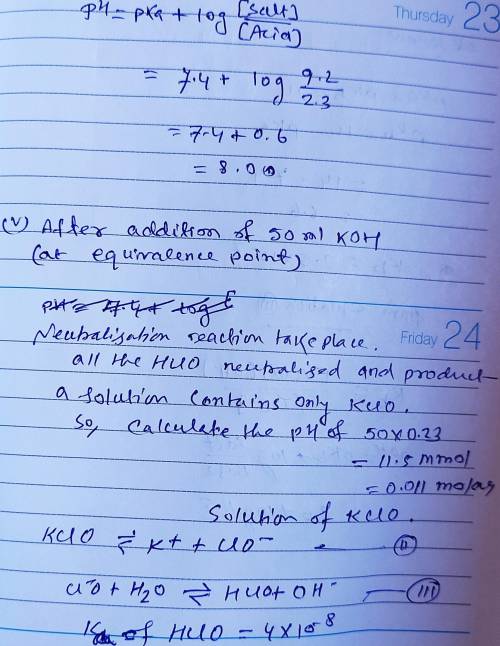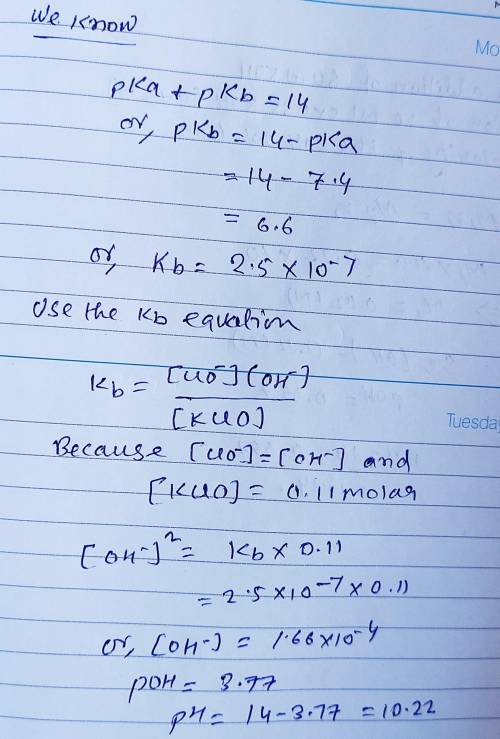Chemistry, 25.03.2020 22:30 eshaesmot12345
Calculate the pH for each case in the titration of 50.0 mL of 0.230 M HClO ( aq ) 0.230 M HClO(aq) with 0.230 M KOH ( aq ) . 0.230 M KOH(aq). Use the ionization constant for HClO . HClO. What is the pH before addition of any KOH ? KOH? pH = pH= What is the pH after addition of 25.0 mL KOH ? 25.0 mL KOH? pH = pH= What is the pH after addition of 30.0 mL KOH ? 30.0 mL KOH? pH = pH= What is the pH after addition of 50.0 mL KOH ? 50.0 mL KOH? pH = pH= What is the pH after addition of 60.0 mL KOH ? 60.0 mL KOH? pH =

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 19:10
Δu of , in kj/kg, as it isto k, (a)as a of , (b) at , (c) at .
Answers: 2

Chemistry, 23.06.2019 01:30
Some molecular compounds, such as hcl, ionize completely in solution. for molecular compounds such as h2co3, most molecules do not ionize in solution. which describes the properties of these two solutes? a. hcl and h2co3 have the same effect on the properties of the solution. b. hcl raises the freezing point of water more than h2co3 does. c. hcl raises the boiling point of water more than h2co3 does.
Answers: 2

Chemistry, 23.06.2019 14:00
How many moles of oxygens atoms are present in 5.00 mol of diphosphorus of fe2(so4)3
Answers: 2

Chemistry, 23.06.2019 15:00
This is a portion of the earths surface that may be far from tectonic plates boundaries yet experiences volcanism due to a rising mantle plume or some other cause
Answers: 3
You know the right answer?
Calculate the pH for each case in the titration of 50.0 mL of 0.230 M HClO ( aq ) 0.230 M HClO(aq) w...
Questions


Mathematics, 14.01.2021 19:10

English, 14.01.2021 19:10




English, 14.01.2021 19:10



History, 14.01.2021 19:10

History, 14.01.2021 19:10


Chemistry, 14.01.2021 19:10


Mathematics, 14.01.2021 19:10



Arts, 14.01.2021 19:10


Arts, 14.01.2021 19:10