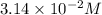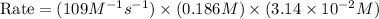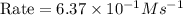Chemistry, 25.03.2020 23:55 markusovaevelyn532
The reaction of nitrogen monoxide with ozone at 25 oC NO + O3NO2 + O2 is first order in NO and first order in O3. Complete the rate law for this reaction in the box below. Use the form k[A]m[B]n... , where '1' is understood for m, n ... (don't enter 1) and concentrations taken to the zero power do not appear. Rate =
In an experiment to determine the rate law, the rate constant was determined to be 109 M-1s-1. Using this value for the rate constant, the rate of the reaction when [NO] = 0.186 M and [O3] = 3.14×10-2 M would be [blank] Ms-1.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which actions would increase the rate at salt dissolves in water? stir the water? crush the salt? use less water? heat the water? cool the salt
Answers: 3

Chemistry, 22.06.2019 05:20
Asolution contains 180 g of glucose (c6h12o6) and 162 g of water. what is the mole fraction of glucose?
Answers: 3

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 09:10
Which class of molecules functions as chemical signals? hormones water carbohydrates proteins
Answers: 1
You know the right answer?
The reaction of nitrogen monoxide with ozone at 25 oC NO + O3NO2 + O2 is first order in NO and first...
Questions

Mathematics, 30.08.2019 13:30



Chemistry, 30.08.2019 13:30

Mathematics, 30.08.2019 13:30


History, 30.08.2019 13:30


History, 30.08.2019 13:30

History, 30.08.2019 13:30

Mathematics, 30.08.2019 13:30


Mathematics, 30.08.2019 13:30

Chemistry, 30.08.2019 13:30



Biology, 30.08.2019 13:30

Health, 30.08.2019 13:30

Mathematics, 30.08.2019 13:30

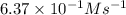
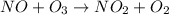
![\text{Rate}=k[NO]^a[O_3]^b](/tpl/images/0564/1543/2796d.png)
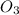 = 1
= 1![\text{Rate}=k[NO]^1[O_3]^1](/tpl/images/0564/1543/85a0b.png)
![\text{Rate}=k[NO][O_3]](/tpl/images/0564/1543/1a568.png)
![[O_3]](/tpl/images/0564/1543/8b13e.png) = concentration of
= concentration of