

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 18:00
Many pharmaceutical drugs are organic compounds that were originally synthesized in the laboratory. which two scientific disciplines are bridged by pharmaceutical drugs? chemistry and forensics chemistry and medicine biology and forensics biology and criminology
Answers: 2

Chemistry, 23.06.2019 05:00
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1

Chemistry, 23.06.2019 10:00
How many moles are equal to 2.4×10^23 formula units of sodium chloride
Answers: 1
You know the right answer?
The rate constant for the reaction is 0.660 M − 1 ⋅ s − 1 0.660 M−1⋅s−1 at 200 ∘ C. 200 ∘C. A ⟶ prod...
Questions



Mathematics, 23.03.2021 18:30

Computers and Technology, 23.03.2021 18:30








Mathematics, 23.03.2021 18:30



Mathematics, 23.03.2021 18:30

Chemistry, 23.03.2021 18:30


Computers and Technology, 23.03.2021 18:30


![k=\frac{1}{t}\left (\frac{1}{[A]}-\frac{1}{[A]_o}\right)](/tpl/images/0564/2226/5ea71.png)
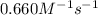
![[A]_o](/tpl/images/0564/2226/9caf5.png) = Initial concentration = 0.00440 M
= Initial concentration = 0.00440 M![0.660=\frac{1}{865}\left (\frac{1}{[A]}-\frac{1}{(0.00440)}\right)](/tpl/images/0564/2226/9d3b6.png)
![[A]=0.00125M](/tpl/images/0564/2226/63ab7.png)


