
At 400 k, the equilibrium constant for the reaction br2 (g) + cl2 (g) 2brcl (g) is kp = 7.0. a closed vessel at 400 k is charged with 1.00 atm of br2 (g), 1.00 atm of cl2 (g), and 2.00 atm of brcl (g). use q to determine which of the statements below is true.
a. the equilibrium partial pressure of br2 will be greater than 1.00 atm.
b. the reaction will go to completion since there are equal amounts of br2 and cl2.
c. the equilibrium partial pressure of brcl (g) will be greater than 2.00 atm.
d. the equilibrium partial pressures of br2, cl2, and brcl will be the same as the initial values.
e. at equilibrium, the total pressure in the vessel will be less than the initial total pressure.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2



Chemistry, 22.06.2019 14:30
What is the relationship between wind and ocean waves? question 17 options: wind moving at higher speeds will transfer more energy to the water, resulting in stronger waves. wind moving at higher speeds will transfer energy over a larger part of the ocean water, resulting in waves with a shorter wavelength. winds moving at higher speeds with cause water to move forward at faster rates, causing larger ocean waves. winds moving at higher speeds will affect deeper water, resulting in waves that move at a faster rate. how do temperature and salinity affect deepwater currents? question 15 options: as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2
You know the right answer?
At 400 k, the equilibrium constant for the reaction br2 (g) + cl2 (g) 2brcl (g) is kp = 7.0. a close...
Questions



Mathematics, 05.10.2019 22:10

History, 05.10.2019 22:10

History, 05.10.2019 22:20

Mathematics, 05.10.2019 22:20






Mathematics, 05.10.2019 22:20



Mathematics, 05.10.2019 22:20

History, 05.10.2019 22:20



History, 05.10.2019 22:20

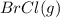 will be greater than
will be greater than 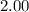 atm.
atm.![Q_{p} =\frac{[products]}{[reactants]}](/tpl/images/0564/6775/77b1b.png)
![Q_{p} = \frac{[2.00]^2}{{[1.00]}[1.00]}](/tpl/images/0564/6775/b1f67.png)
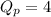
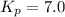
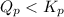 .
.


