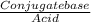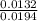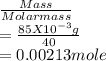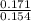Chemistry, 26.03.2020 05:19 kkjones1536
A 100.0-mL buffer solution is 0.175 M in HClO and 0.150 M in NaClO. Part A: What is the initial pH of this solution?Part B: What is the pH after addition of 150.0 mg of HBr?Part C: What is the pH after addition of 85.0 mg of NaOH?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
50 pts plz what is the physical state of matter of baking soda.
Answers: 1

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 23.06.2019 05:00
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1

Chemistry, 23.06.2019 08:00
Pl what kind of reaction is this? nahco3 + h2o → co2 + naoh + h2o -composition -decomposition -single replacement -double replacement im leaning more toward single replacement. if im wrong can you explain whyy?
Answers: 2
You know the right answer?
A 100.0-mL buffer solution is 0.175 M in HClO and 0.150 M in NaClO. Part A: What is the initial pH o...
Questions

Arts, 13.07.2019 20:00

Mathematics, 13.07.2019 20:00



Social Studies, 13.07.2019 20:00

Mathematics, 13.07.2019 20:00

History, 13.07.2019 20:00







Mathematics, 13.07.2019 20:00

Chemistry, 13.07.2019 20:00





Mathematics, 13.07.2019 20:00

![\frac{[Conjugate base]}{[Acid]}](/tpl/images/0564/8323/225c1.png)
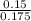
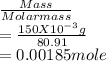
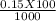 = 0.015
= 0.015 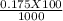 = 0.0175
= 0.0175