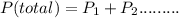Chemistry, 26.03.2020 16:46 manarsadi6
1. A sample of oxygen is collected over water at 22 ° C and 762 torr. What is the partial pressure of the dry oxygen? The vapor pressure of water at 22°C is 19.8 torr. A. 742 torr B. 782 torr C. 784 torr D. 750. Torr

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 15:00
What is the most important factor in determining climates.
Answers: 1
You know the right answer?
1. A sample of oxygen is collected over water at 22 ° C and 762 torr. What is the partial pressure o...
Questions

History, 01.07.2019 04:00




Mathematics, 01.07.2019 04:00


Mathematics, 01.07.2019 04:00

Mathematics, 01.07.2019 04:00

History, 01.07.2019 04:00

Health, 01.07.2019 04:00






Biology, 01.07.2019 04:00



English, 01.07.2019 04:00