

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
10-14. (a) when 100.0 ml of weak acid ha were titrated with 0.093 81 m naoh, 27.63 ml were required to reach the equivalence point. find the molarity of ha. (b) what is the formal concentration of a- at the equivalence point? (c) the ph at the equivalence point was 10.99. find pk. for ha. (d) what was the ph when only 19.47 ml of naoh had been added?
Answers: 1


Chemistry, 22.06.2019 06:00
The tilt of the earth's axis of rotation is responsible for the a) ocean's tides. b) size of the moon. c) brightness of stars. d) earth’s seasons.
Answers: 1

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1
You know the right answer?
How many moles of fe2o3 will be produced from 18.0 g of fe assuming o2 is available in excess...
Questions

Mathematics, 07.04.2021 21:50


Mathematics, 07.04.2021 21:50

Mathematics, 07.04.2021 21:50


Chemistry, 07.04.2021 21:50


Physics, 07.04.2021 21:50

Mathematics, 07.04.2021 21:50




Arts, 07.04.2021 21:50

Chemistry, 07.04.2021 21:50

Mathematics, 07.04.2021 21:50

Mathematics, 07.04.2021 21:50



Chemistry, 07.04.2021 21:50

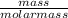
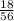 = 0.32mole
= 0.32mole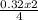 = 0.161 moles of Fe₂O₃
= 0.161 moles of Fe₂O₃


