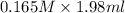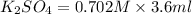Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1

Chemistry, 22.06.2019 16:10
Given the following equation: 2a1 + 3mgcl2 --> 2alcl3 + 3mg how many moles of aluminum chloride are produced from 2.5 moles of magnesium chloride?
Answers: 1

Chemistry, 22.06.2019 16:40
Let the ed50 of a recreational drug be defined as the amount required for 50% of a test group to feel high or get a buzz. if the ed50 value of ethanol is 470 mg/kg body mass, what dose would a 70 kg party goer need to quickly consume in order to have a 50% chance of getting a buzz? 235 mg 470 mg 32,900 mg 35,000,000 mg
Answers: 3
You know the right answer?
When aqueous solutions of K2SO4 and Pb(NO3)2 are combined, PbSO4 precipitates. Calculate the mass, i...
Questions


Mathematics, 13.10.2020 14:01



History, 13.10.2020 14:01

Social Studies, 13.10.2020 14:01


Advanced Placement (AP), 13.10.2020 14:01

Mathematics, 13.10.2020 14:01

Mathematics, 13.10.2020 14:01



English, 13.10.2020 14:01

Biology, 13.10.2020 14:01

Mathematics, 13.10.2020 14:01


History, 13.10.2020 14:01

Mathematics, 13.10.2020 14:01


History, 13.10.2020 14:01

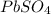 produced is 99.1 g.
produced is 99.1 g.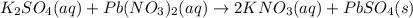
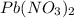 = Molarity × volume
= Molarity × volume