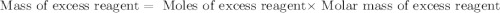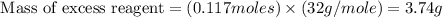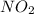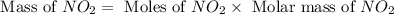Chemistry, 26.03.2020 19:41 Hilljos018
For this reaction, 11.5 g nitrogen monoxide reacts with 9.91 g oxygen gas. nitrogen monoxide (g) + oxygen (g) nitrogen dioxide (g) What is the maximum mass of nitrogen dioxide that can be formed? g What is the FORMULA for the limiting reagent? What mass of the excess reagent remains after the reaction is complete?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:00
When you mate two plants together the terms is called? answer it fast as possible plz! i have a test tomorrow!
Answers: 1

Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
For this reaction, 11.5 g nitrogen monoxide reacts with 9.91 g oxygen gas. nitrogen monoxide (g) + o...
Questions

History, 09.12.2020 21:00


Chemistry, 09.12.2020 21:00







Mathematics, 09.12.2020 21:00

English, 09.12.2020 21:00

Mathematics, 09.12.2020 21:00



Mathematics, 09.12.2020 21:00

Mathematics, 09.12.2020 21:00

Chemistry, 09.12.2020 21:00

Mathematics, 09.12.2020 21:00


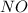
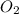 = 9.91 g
= 9.91 g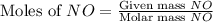
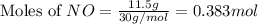
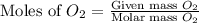
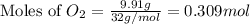
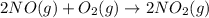
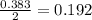 moles of
moles of