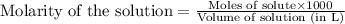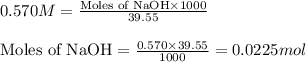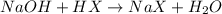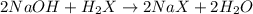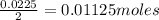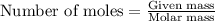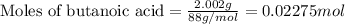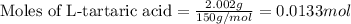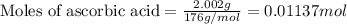Chemistry, 26.03.2020 20:02 taridunkley724
A student is given a 2.002 g sample of unknown acid and is told that it might be butanoic acid, a monoprotic acid (HC4H7O2, equation 1), L-tartaric acid, a diprotic acid (H2C4H4O6, equation 2), or ascorbic acid, a diprotic acid (H2C6H6O6, equation 3). If it requires 39.55 mL of 0.570 M NaOH(aq) to neutralize the unknown acid, what is the identity of the unknown acid

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
How has the scientific community addressed the safety of chemicals? a. chemicals are repeatedly tested, even those that have existed for a long time. b. existing chemicals are tested if they have never been tested before. c. chemicals are tested if they are suspected to have caused a problem. d. only new chemicals are tested.
Answers: 2

Chemistry, 23.06.2019 00:30
Fred is studying a substance that is made out of only one element. this means that
Answers: 1

Chemistry, 23.06.2019 01:00
How does carbon monoxide pose the greatest threat to humans? a. it can be produced by wood fires. b. it can be produced by home furnaces. c. it is produced by acid rain. d. it is produced by modern automobiles.
Answers: 2

You know the right answer?
A student is given a 2.002 g sample of unknown acid and is told that it might be butanoic acid, a mo...
Questions



Physics, 22.06.2019 16:00


Mathematics, 22.06.2019 16:00




Mathematics, 22.06.2019 16:00





Geography, 22.06.2019 16:00




Physics, 22.06.2019 16:00