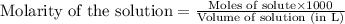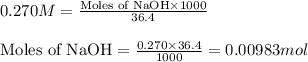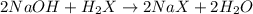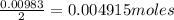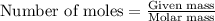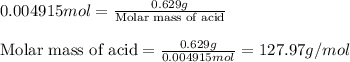A 0.629 g sample of a diprotic acid is dissolved in water and titrated with 0.270 M NaOH. What is the molar mass of the acid if 36.4 mL of the NaOH solution is required to neutralize the sample? Assume the volume of NaOH corresponds to the second equivalence point. A flask with a solution sits on the base of a ring stand. A buret filled with liquid is suspended above the flask by the ring stand. molar mass: g/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
You know the right answer?
A 0.629 g sample of a diprotic acid is dissolved in water and titrated with 0.270 M NaOH. What is th...
Questions


Health, 28.01.2020 13:52


Computers and Technology, 28.01.2020 13:52

Biology, 28.01.2020 13:52

English, 28.01.2020 13:52



Social Studies, 28.01.2020 13:52

World Languages, 28.01.2020 13:52

Mathematics, 28.01.2020 13:52




Mathematics, 28.01.2020 13:52

Biology, 28.01.2020 13:52

Mathematics, 28.01.2020 13:52

History, 28.01.2020 13:52

Social Studies, 28.01.2020 13:52

Mathematics, 28.01.2020 13:52