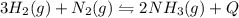In the reaction represented by the equation n2(g) + 3h2(g) 2nh3(g), the
forward reaction is ex...

Chemistry, 10.10.2019 16:30 jacobbecker99
In the reaction represented by the equation n2(g) + 3h2(g) 2nh3(g), the
forward reaction is exothermic. which set of conditions would give the best yield of
ammonia at equilibrium?
a) 800 atmospheres and 2000 oc
b) 1 atmosphere and 500 o
c
c) 1 atmosphere and 2000 o
c
d) 800 atmospheres and 500 o
c

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Identify two types of chemical bonding in the source of dietary potassium
Answers: 3

Chemistry, 22.06.2019 16:10
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.300 m , [b] = 1.05 m , and [c] = 0.550 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.140 m and [c] = 0.710 m . calculate the value of the equilibrium constant, kc.
Answers: 1


You know the right answer?
Questions





Mathematics, 26.03.2021 18:20

Mathematics, 26.03.2021 18:20

Mathematics, 26.03.2021 18:20

Mathematics, 26.03.2021 18:20

English, 26.03.2021 18:20

Chemistry, 26.03.2021 18:20


History, 26.03.2021 18:20

Mathematics, 26.03.2021 18:20

Social Studies, 26.03.2021 18:20

Mathematics, 26.03.2021 18:20


Health, 26.03.2021 18:20

Chemistry, 26.03.2021 18:20

Mathematics, 26.03.2021 18:20