
Chemistry, 26.03.2020 20:48 rubycarbajal
The explosive nitroglycerin (C3H5N3O9) decomposes rapidly upon ignition or sudden impact according to the following balanced equation:
4C3H5N3O9(l)→12CO2(g)+10H2O(g)+6N2( g)+O2(g) ΔH∘rxn=−5678kJ
Required:
Calculate the standard enthalpy of formation (ΔH∘f) for nitroglycerin.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which sentence best describes the formation of igneous rock? a- lava on the surface dries up and makes arock b_melted rocks cools and forms crystals c_rocks under tremendous heat and pressure d_magma is melted rock underground
Answers: 1

Chemistry, 22.06.2019 10:30
How do you lengthen a pattern piece? (family and consumer science, sewing)
Answers: 2

Chemistry, 22.06.2019 22:30
Vi limitens. vastery test select the correct answer. which statement explains why large atoms are more reactive than small atoms? a. large atoms have valence electrons farther from the nucleus and lose them more readily. b. large atoms have greater ionization energy, which they can utilize during a reaction. c. large atoms have a greater number of electrons that they can lose during a reaction. d. large atoms have more energy levels, so they have more energy to pass on in a reaction. reset next
Answers: 3

You know the right answer?
The explosive nitroglycerin (C3H5N3O9) decomposes rapidly upon ignition or sudden impact according t...
Questions


English, 21.11.2020 04:20



Mathematics, 21.11.2020 04:20

English, 21.11.2020 04:20

Mathematics, 21.11.2020 04:20

Mathematics, 21.11.2020 04:20


Mathematics, 21.11.2020 04:20

Mathematics, 21.11.2020 04:20

English, 21.11.2020 04:20



Mathematics, 21.11.2020 04:20

Geography, 21.11.2020 04:20



Mathematics, 21.11.2020 04:20

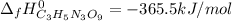
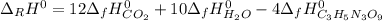
![\Delta _fH^0_{C_3H_5N_3O_9}=\frac{1}{4} (12\Delta _fH^0_{CO_2}+10\Delta _fH^0_{H_2O}-\Delta _RH^0)\\\Delta _fH^0_{C_3H_5N_3O_9}=\frac{1}{4mol} [12(-393.5kJ)+10(-241.8kJ)-(-5678kJ)]\\\Delta _fH^0_{C_3H_5N_3O_9}=-365.5kJ/mol](/tpl/images/0565/7176/fdf74.png)


