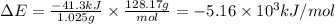Chemistry, 26.03.2020 20:54 Gearyjames8
Mothballs are composed primarily of naphthalene (C10H8). When 1.025 g of naphthalene burns in a bomb calorimeter, the temperature rises from 24.25 C to 32.33 C. (The Heat Capacity of the Calorimeter is 5.11 kJ/C.) Find DeltaE (in x 103 kJ) for the combustion of naphthalene.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1

Chemistry, 23.06.2019 01:00
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1

Chemistry, 23.06.2019 04:31
One student said that the investigation was not valid (a fair test). write a plan for the investigation that includes improvements to the method and apparatus
Answers: 1
You know the right answer?
Mothballs are composed primarily of naphthalene (C10H8). When 1.025 g of naphthalene burns in a bomb...
Questions


Chemistry, 05.02.2021 22:30

English, 05.02.2021 22:30

Mathematics, 05.02.2021 22:30

Computers and Technology, 05.02.2021 22:30

Mathematics, 05.02.2021 22:30


History, 05.02.2021 22:30




English, 05.02.2021 22:30



Mathematics, 05.02.2021 22:30

Mathematics, 05.02.2021 22:30

Chemistry, 05.02.2021 22:30

Mathematics, 05.02.2021 22:30


English, 05.02.2021 22:30