
Problem PageQuestion Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide and gaseous water . Suppose 10. g of octane is mixed with 61.9 g of oxygen. Calculate the minimum mass of octane that could be left over by the chemical reaction. Round your answer to significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3

Chemistry, 22.06.2019 19:30
Estimate the molar mass of the gas that effuses at 1.6 times the effusion rate of carbon dioxide.
Answers: 1

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 22.06.2019 21:30
Which of the following changes will decrease the total amount of gaseous solute able to be dissolved in a liter of liquid water? (2 points) decreasing temperature decreasing pressure decreasing surface area decreasing solute concentration
Answers: 1
You know the right answer?
Problem PageQuestion Liquid octane will react with gaseous oxygen to produce gaseous carbon dioxide...
Questions

Mathematics, 23.04.2020 19:25

Mathematics, 23.04.2020 19:25

Mathematics, 23.04.2020 19:25





Chemistry, 23.04.2020 19:25


Mathematics, 23.04.2020 19:25

Mathematics, 23.04.2020 19:25




Mathematics, 23.04.2020 19:25


Social Studies, 23.04.2020 19:25



Mathematics, 23.04.2020 19:26

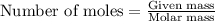
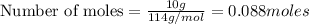
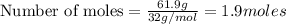
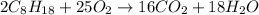
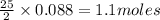 of ethane
of ethane

