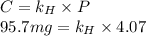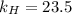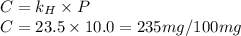Chemistry, 26.03.2020 22:40 xxgissellexx
At a certain temperature, the solubility of N2 gas in water at 4.07 atm is 95.7 mg of N2 gas/100 g water . Calculate the solubility of N2 gas in water, at the same temperature, if the partial pressure of N2 gas over the solution is increased from 4.07 atm to 10.0 atm .

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3
You know the right answer?
At a certain temperature, the solubility of N2 gas in water at 4.07 atm is 95.7 mg of N2 gas/100 g w...
Questions


History, 20.07.2019 02:30

History, 20.07.2019 02:30


History, 20.07.2019 02:30



History, 20.07.2019 02:30



History, 20.07.2019 02:30


English, 20.07.2019 02:30

Mathematics, 20.07.2019 02:30

History, 20.07.2019 02:30

English, 20.07.2019 02:30

History, 20.07.2019 02:30


Health, 20.07.2019 02:30

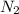 gas in water, at the same temperature, if the partial pressure of gas is 10.0 atm is 235mg/100g.
gas in water, at the same temperature, if the partial pressure of gas is 10.0 atm is 235mg/100g.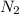 in water can be calculated by Henry’s Law. Henry’s law gives the relation between gas pressure and the concentration of dissolved gas.
in water can be calculated by Henry’s Law. Henry’s law gives the relation between gas pressure and the concentration of dissolved gas.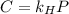 .
.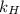 = Henry’s law constant = ?
= Henry’s law constant = ?