
Chemistry, 26.03.2020 22:42 elijahsantiago21
Calculate the number of grams of CH3COONa * 3H2O (sodium acetate tri-hydrate) needed to make 250.0 mL of a CH3COOH (acetic acid)/ CH3COONa * 3H2O buffer. The target pH of the buffer is 5.25. The given concentration of [CH3COOH] is equal to 0.10 M. Ka = 1.80 x 10-5 for acetic acid.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 2

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

You know the right answer?
Calculate the number of grams of CH3COONa * 3H2O (sodium acetate tri-hydrate) needed to make 250.0 m...
Questions

Spanish, 01.09.2019 05:10

Mathematics, 01.09.2019 05:10

English, 01.09.2019 05:10


Mathematics, 01.09.2019 05:10

History, 01.09.2019 05:10

English, 01.09.2019 05:10


English, 01.09.2019 05:10





History, 01.09.2019 05:10




History, 01.09.2019 05:10

Mathematics, 01.09.2019 05:10

History, 01.09.2019 05:10

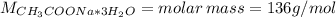
![pH = pKa + log(\frac{[CH_{3}COONa*3H_{2}O]}{[CH_{3}COOH]})](/tpl/images/0566/0820/ec252.png) (1)
(1)![log [CH_{3}COONa*3H_{2}O] = pH - pKa + log [CH_{3}COOH]](/tpl/images/0566/0820/d2774.png)
![log [CH_{3}COONa*3H_{2}O] = 5.25 - (-log(1.80 \cdot 10^{-5})) + log (0.10) = -0.495](/tpl/images/0566/0820/9589c.png)
![[CH_{3}COONa*3H_{2}O] = 10^{-0.495} = 0.32 M](/tpl/images/0566/0820/c6ecf.png)
![m = moles*M = [CH_{3}COONa*3H_{2}O]*V*M = 0.32 mol/L*0.250 L*136 g/mol = 10.88 g](/tpl/images/0566/0820/427f5.png)


