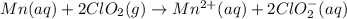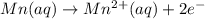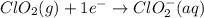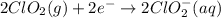Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:20
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 01:40
Which characteristic of water it form droplets? a. low specific heat b. nonpolar structure c. high surface tension d. ability to dissolve substances
Answers: 1

Chemistry, 22.06.2019 17:40
How much heat is added if 0.814g of water increase in temperature by 0.351 degree c?
Answers: 3

Chemistry, 22.06.2019 21:30
How many oxygen atoms are there in 3.15 moles of hcl manganese (iv) oxide, mno2
Answers: 2
You know the right answer?
Write the overall balanced equation occurring in a cell that has an anode consisting of a Mn(s) elec...
Questions


Spanish, 19.04.2021 21:30




Mathematics, 19.04.2021 21:30

Mathematics, 19.04.2021 21:30

Mathematics, 19.04.2021 21:30

Mathematics, 19.04.2021 21:30



English, 19.04.2021 21:30

Mathematics, 19.04.2021 21:30

Geography, 19.04.2021 21:30

Spanish, 19.04.2021 21:30


Mathematics, 19.04.2021 21:30

Mathematics, 19.04.2021 21:30

Mathematics, 19.04.2021 21:30

History, 19.04.2021 21:30