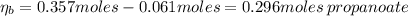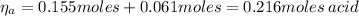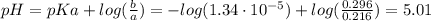A 1.41 L buffer solution consists of 0.253 M propanoic acid and 0.110 M sodium propanoate. Calculate the pH of the solution following the addition of 0.061 mol HCl . Assume that any contribution of the HCl to the volume of the solution is negligible. The K a of propanoic acid is 1.34 × 10 − 5 .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
What type of electromagnetic radiation has a shorter wavelength than blue light
Answers: 2

Chemistry, 22.06.2019 08:30
What method(s) do plants use to obtain nitrogen? select all that apply. absorb it from the atmosphere use bacteria to convert nitrogen to usable form obtain usable nitrogen compounds from the soil absorb nitrogen from water taken in at the roots
Answers: 3

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 23.06.2019 05:00
How is electrolysis most commonly used to produce an energy source? a - splitting water molecules produces oxygen, which organisms breathe to fuel their bodies. b - splitting water molecules produces hydrogen gas, which is used to power machines through hydrogen fuel cells. c - splitting carbon dioxide molecules produces coal, a form of carbon that can be burned to produce heat. d - splitting carbon dioxide molecules produces natural gas, which can be burned to generate electricity in power plants.
Answers: 1
You know the right answer?
A 1.41 L buffer solution consists of 0.253 M propanoic acid and 0.110 M sodium propanoate. Calculate...
Questions

Mathematics, 03.05.2021 20:00

Social Studies, 03.05.2021 20:00

World Languages, 03.05.2021 20:00

Mathematics, 03.05.2021 20:00


Mathematics, 03.05.2021 20:00

Mathematics, 03.05.2021 20:00



Chemistry, 03.05.2021 20:00

History, 03.05.2021 20:00


Computers and Technology, 03.05.2021 20:00







Geography, 03.05.2021 20:00

![\eta_{a} = [a]*Va = 0.110 M * 1.41 L = 0.155 moles \thinspace acid](/tpl/images/0566/2281/d601e.png)
![\eta_{b} = [b]*Vb = 0.253 M * 1.41 L = 0.357 moles\thinspace propanoate](/tpl/images/0566/2281/b75d2.png)